Packaging Size 1 x 10 tablet
Rybelsus: Control Your Diabetes and Lose Weight Without Using A Needle

In September 2019, the U.S. Food and Drug Administration (FDA) approved Novo Nordisk’s oral tablets Rybelsus (semaglutide). Rybelsus, along with diet and exercise can help improve control of blood sugar in adult patients with type 2 diabetes.
According to the FDA’s information, Rybelsus is the first glucagon-like peptide (GLP-1) receptor protein treatment approved for use in the United States that does not need to be injected.
GLP-1 drugs are non-insulin treatments for people with type 2 diabetes.
Rybelsus has the same active ingredient, semaglutide, as Novo’s Ozempic.
In December 2017, the FDA approved Ozempic injection 0.5 mg or 1 mg, a once-weekly GLP-1 receptor agonist indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes.
How The GLP-1 Agonists Work
GLP-1 is a hormone that is released when we eat. Its job is to stimulate insulin secretion and inhibit glucagon, the hormone that notifies the liver to produce glucose. GLP-1 agonists are able to copy the action of the hormone and lower blood sugar.
In addition, GLP-1 agonists slow the rate that food moves through the digestive tract, leading to a feeling of fullness. All these actions occur when glucose is present, so the risk of hypoglycemia is low with all GLP-1 agonists.
Blood Sugar Control and Weight Loss

As a result of above actions, many people lower their HbA1C, a marker of blood sugar control, 1-1.5% with these drugs. Some have seen an even greater reduction.
Due to the action of food not leaving the stomach as quickly, having a suppressed appetite has people eating less overall. Patients in studies experienced weight loss ranging from 4 -10 pounds over the year-long duration.
More significant amounts of weight loss can be seen, but it varies with the level of changes in diet and physical activity that is implemented as well.

Heart Protective Benefits
On Jan 16, 2020, Novo Nordisk announced that the FDA had approved a new indication for Ozempic® (semaglutide) injection 0.5 mg or 1 mg to reduce the risk of major adverse cardiovascular events (MACE) such as heart attack, stroke, or death in adults with type 2 diabetes and known heart disease.
The FDA has also added data to the label for Rybelsus, confirming that it is safe for the heart.
Rybelsus Vs. Ozempic
You may wonder how Rybelsus compares with other medications that are prescribed for similar uses. Here is data from comparing how Rybelsus and Ozempic are alike and how they are different.
Ingredients
Both Rybelsus and Ozempic contain the active ingredient semaglutide.
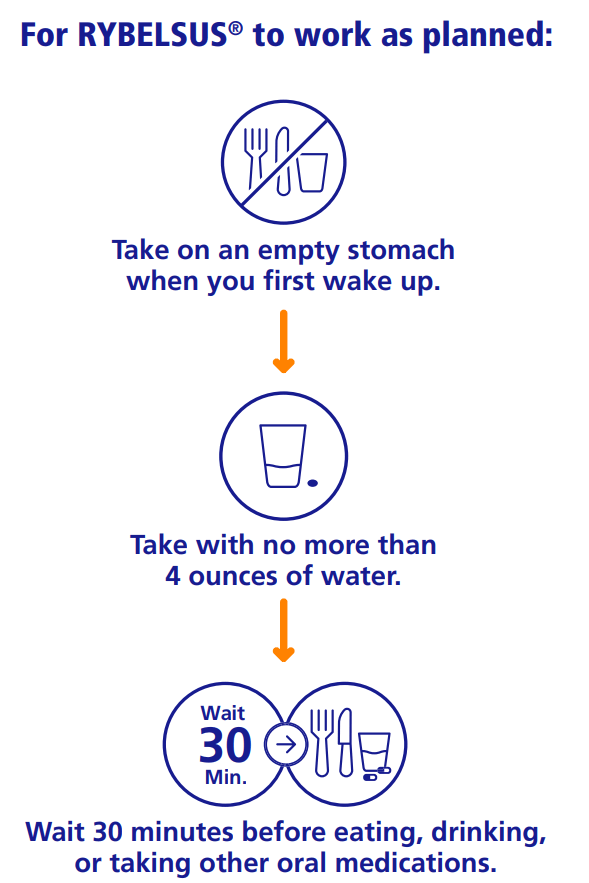
Uses
Rybelsus and Ozempic aren’t approved to treat type 1 diabetes or a complication of diabetes called diabetic ketoacidosis.
Also, the drugs haven’t been studied in people who have had a pancreas problem known as pancreatitis.
If diet and exercise aren’t helping your type 2 diabetes, Rybelsus shouldn’t be the first medication that you try.
Drug Forms and Administration
- Rybelsus comes as a tablet that you swallow.
- Ozempic comes as a single-use pen that you use to give yourself an injection just under your skin (subcutaneous). You’ll have the injections in your belly, thigh, or upper arm.










Reviews
There are no reviews yet.